From the site landing page the default view will list all sites enrolled in the study. To create a subject, select the site to which subjects will be added by clicking on the underlined site name shown in Figure 1 below.
If there are no subjects yet entered, the screen will look like Figure 2 below. Action links are in the side bar as shown.
Figure 1: Site Landing Page - Populated
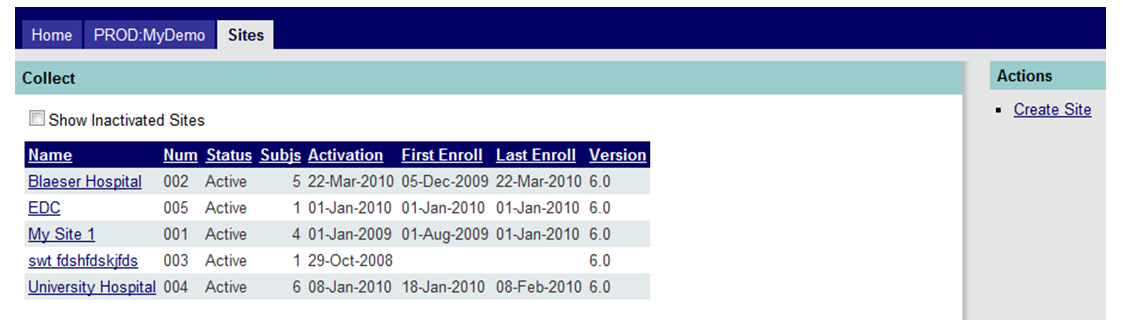
Figure 2: Site/Subject Page - Unpopulated
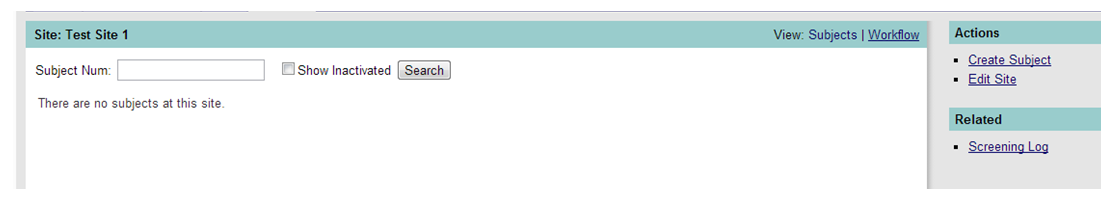
The list of possible actions (as shown in Figure 2 above) shows a site that is in an “Active” status, thus the “Create Subject” link is enabled (underlined).
When a site has not yet been activated, the “Create Subject” link is not enabled (not underlined) as in Figure 3 below.
Figure 3: Create Subject Link - Disabled
As you can see in Figure 2 above, the Screening Log may be visible (Related items in the sidebar) if the sponsor has chosen to activate this feature. If you wish to screen patients as potential subjects, please refer to the Screening Log section.
To add a subject, click on the Create Subject link. Figure 4 below shows the fields available on this screen with a description of the fields which follow.
Figure 4: Create Subject
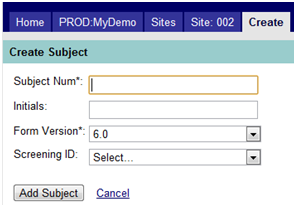
Subject Num |
Subject number, which should be unique within the study. This number may be assigned manually or the user may employ the auto-numbering feature. If auto-numbering is enabled (at the request of the sponsor or program manager), the next subject number for that site will auto-populate this field when the form is opened. The user may override this number if desired and enter another unique ID. [Note: If auto-numbering is enabled and a user re-numbers a subject skipping numbers in the sequence, MAESTRO will look for the highest number (even if numbers were skipped) and increase that number by one for the next number in the series. Users may need to look for missing numbers to manually assign subjects so there are no gaps.] Most studies will use a combination of the site number (e.g. 002) followed by a sequence number increasing incrementally by one (e.g., 001, 002, 003), so all subjects at a particular site begin with the same digits. If a 3-digit site number is assigned, this would result in a six-digit subject number: 002-001. [Note: If the user attempts to manually assign a subject number that has already been assigned to another subject, the following error message will appear: “The subject number must be unique within the study”.] If the trial sponsor chooses to use the auto-numbering feature, they will identify the sequencing they wish to use which will be programmed into MAESTRO by Libra staff. |
Initials |
Three letter subject initials (usually first, middle, last). |
Form Version |
The latest version of the database associated with that subject. [Note: The user will typically accept the default value in this field, based on the value assigned in the “Study” form. The form (database) version always maps to a protocol revision/version. If the protocol changes mid-study, you may see: a subject with CRFs under different form (database) versions; and different form (database) version between subjects.] |
Screening ID |
If applicable, choose the ID assigned in the screening log. |
Click the “Add Subject” button to save the subject data or Cancel to return to the previous screen without saving the data. At this time, a default set of CRFs, identified by the trial’s sponsor or project manager, is also created for the subject.