If sites have been previously entered, you’ll see the sites landing page like the one in Figure 1 below.
Figure 1: All Sites Landing Page
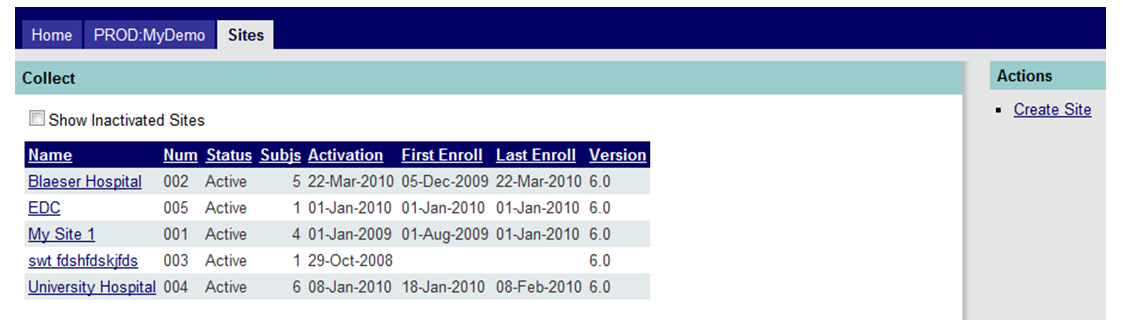
In this screen (Sites tab) a number of columns are visible with data that summarizes each investigative site. See the table below.
Name |
The name of the site. |
Num |
The site number, if one has been assigned. |
Status |
The current status of the site (Candidate, Qualified, Waiting IRB, Active, Suspended, Closed). |
Subjs |
The number of active subjects at the site. |
Activation |
Date of site’s activation. |
First Enroll |
Date of first subject’s enrollment. |
Last Enroll |
Date of last subject’s enrollment. |
Version |
The version of the database currently used at a site. |
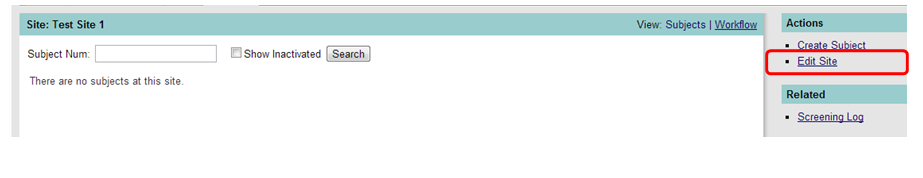
To edit a site, click on the underlined name of the site which contains a hyperlink that will navigate you to form for that site. To edit the site, click on Edit Site under Actions in the side bar. See Figure 2 below.
Figure 2: Actions: Edit Site
There are additional fields in the Edit Site screen that are available when the site is created. See Figure 3 below.
Figure 3: Edit Site Screen
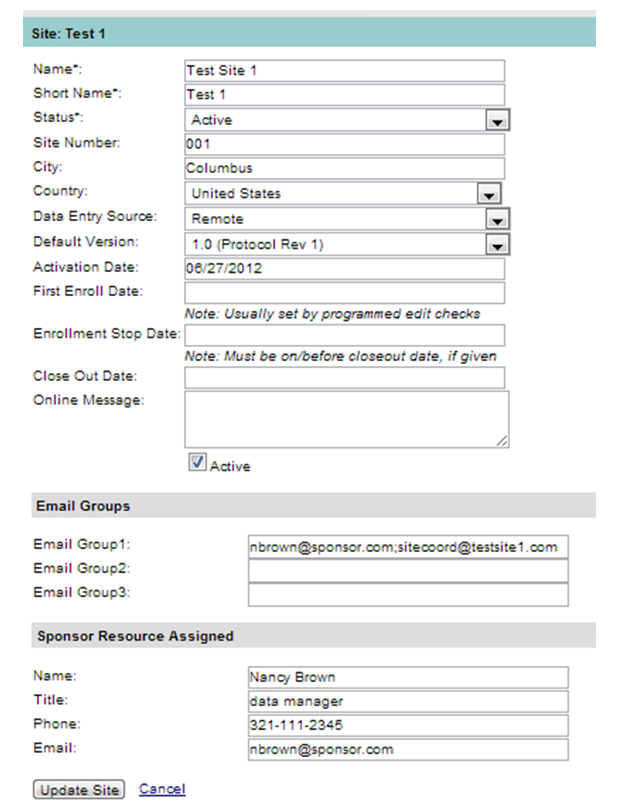
Additional fields/sections below are available in the Edit Site mode that are not available in the Create Site mode.
First Enroll Date |
The date the first subject was enrolled. |
Enrollment Stop Date |
The date enrollment was discontinued at the site. |
Close Out Date |
The date the site was considered closed. |
Online Message |
This message will appear at the top of the site landing page. It remains active until removed from this field. |
Email Group Section |
Email addresses of individuals assigned to groups for email notifications. An unlimited number of email may be entered, separated by a comma. (Email groups may be set up for the purpose of notifying the parties of pertinent study events, such as the enrollment of a subject, reporting of an adverse event or protocol deviation. There are three potential email groups available that could be assigned to an event.) |
Sponsor Resource |
Main sponsor contact for the clinical trial. |