Step 1: Navigate to the Procedure/Treatment Case Report Form (or applicable CRF where the device information is accessed). This CRF is accessed from the Collect Module, Site Name, Subject and Form hyperlinks. Find that portion of the CRF that applies to the recording of product/devices. That portion may be similar in appearance to the section shown in Figure 1 below, but is customized for each clinical trial.
Figure 1: Product Disposition – Assigned via the Procedure (or similar) CRF
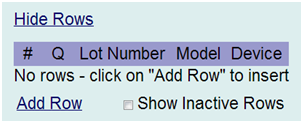
Step 2: Click Add Row. In the next form that appears (example shown in Figure 2 below), you will enter product information associated with that subject.
Figure 2: Product Assignment Form
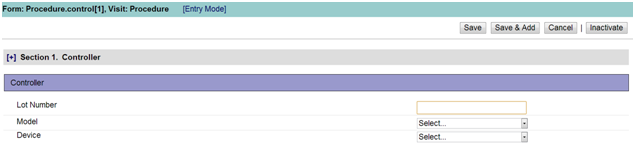
Enter data in the fields described below: [Note: This is a sample form. Each clinical trial will have its own custom set of fields in this form.]
match exactly the info on the Product page in order to register--include
letters, dashes, spaces, etc. if they appear).
Step 3: When finished, click “Save” or “Save & Add” to additional products.